Relè Solenoide Di Avviamento Per Yamaha FZ6 / FZ600 2004-2009 - Nuovo, Marca Caltric
- Corriere espressoda domenica 8 marzoGRATIS

- Informazioni legali
- Ne hai uno da vendere?
- 493668
- 15777735813
Pensati per Te
Prodotti simili
Descrizione
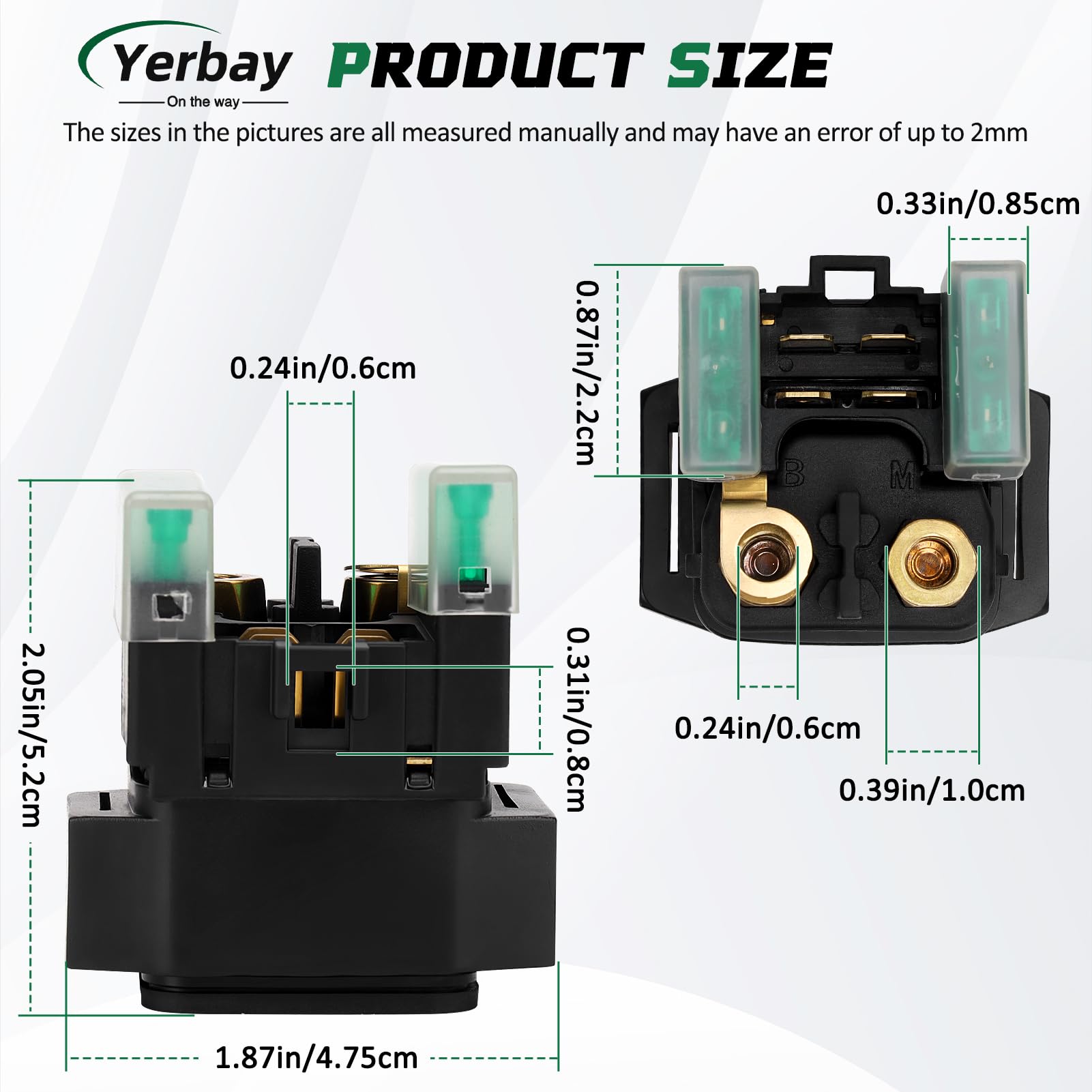
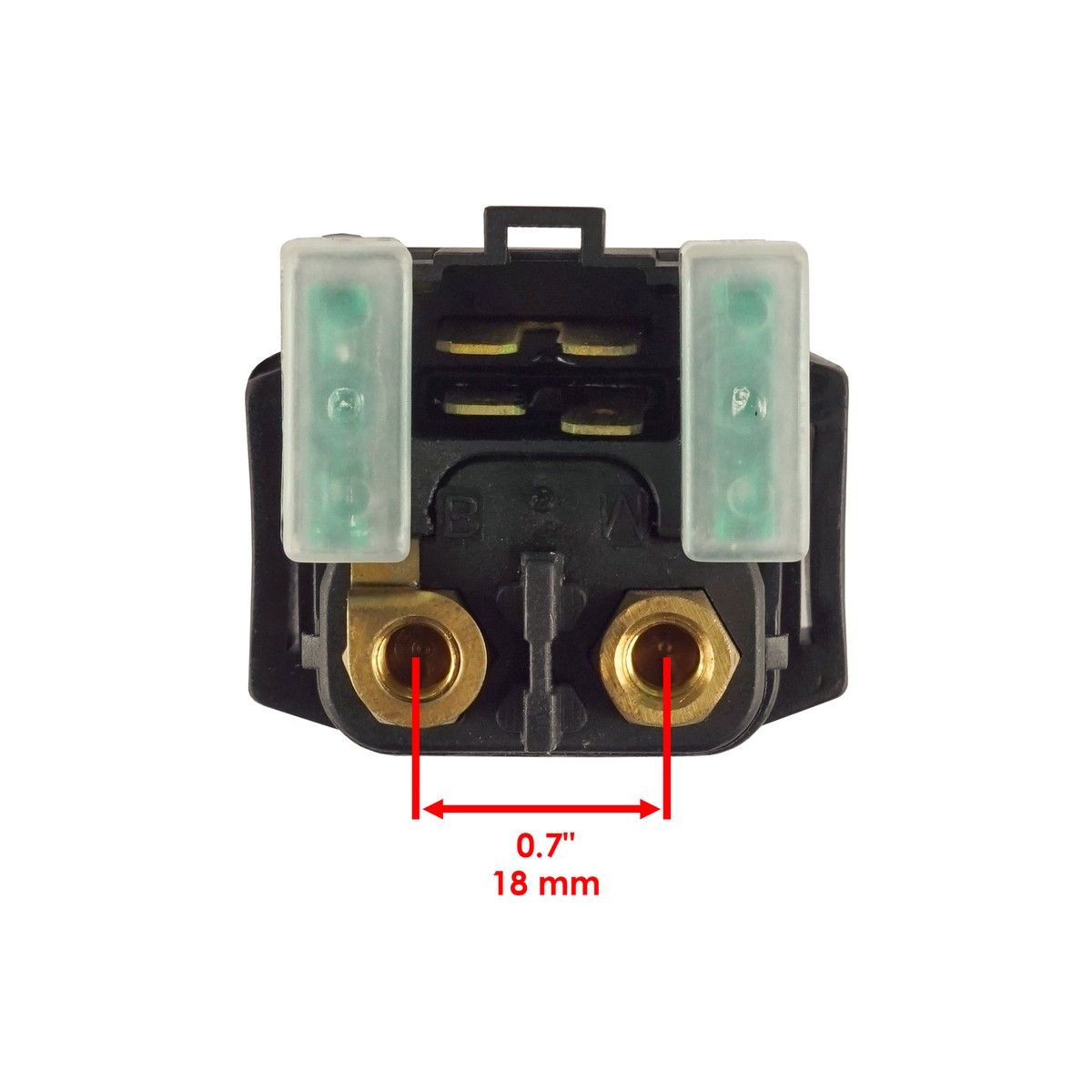
Questo relè solenoide dell’avviamento è nuovo, della marca Caltric. Compatibile con Yamaha FZ600 / FZ6 degli anni 2004-2009. Sostituisce i numeri OEM originali come 1D0-81940, 4BH-81940, 4SV-81940. Realizzato in accia e rame, quindi buona conduzione elettrica. Nella confezione trovi solo il relè (1 pezzo). Ha una garanzia del produttore di 90 giorni. Prodotto in Cina. Attenzione: per i consumatori della California, c’è l’avvertimento Proposition 65 (sostanze chimiche). Questo componente è fondamentale: quando giri la chiave, è lui che manda la corrente grossa al motorino di avviamento. Se la tua moto non parte ma senti solo un “clic” o nulla, potrebbe essere guasto il solenoide. Facile da sostituire, di solito vicino alla batteria. Un ricambio aftermarket economico e funzionale. Verifica la compatibilità con il tuo modello e anno esatto. UPC non applicabile.
Caratteristiche e scheda tecnica
Caratteristiche principali
- EAN3632221029880